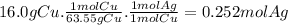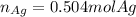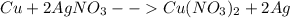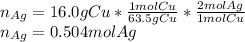Given the following reaction: \ce{cu + 2agno3 -> 2ag + cu(no3)2}cu+2agno3 2ag+cu(nox 3 )x 2 how many moles of \ce{ag}aga, g will be produced from 16.0 \text{ g}16.0 g16, point, 0, start text, space, g, end text of \ce{cu}cuc, u, assuming \ce{agno3}agno3 is available in excess

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2

Chemistry, 23.06.2019 00:20
How many lone pairs of electrons are on the central atom of no3- and what is the molecular shape? one, trigonal planar zero, trigonal pyramidal zero, trigonal planar one, tetrahedral one, trigonal pyramidal
Answers: 1

Chemistry, 23.06.2019 11:50
How many moles of an ideal gas would occupy a 25.0 liter container when the temperature is 295 k and the pressure is 0.850 atm?
Answers: 2

Chemistry, 23.06.2019 17:40
Which type of bonding involves the complete transfer of a valence electron from a less electronegative atom to a more electronegative one?
Answers: 1
You know the right answer?
Given the following reaction: \ce{cu + 2agno3 -> 2ag + cu(no3)2}cu+2agno3 2ag+cu(nox 3 )x 2...
Questions



Mathematics, 20.05.2021 20:30

Mathematics, 20.05.2021 20:30

English, 20.05.2021 20:30




Advanced Placement (AP), 20.05.2021 20:30

History, 20.05.2021 20:30

Mathematics, 20.05.2021 20:30

Spanish, 20.05.2021 20:30

Biology, 20.05.2021 20:30

Mathematics, 20.05.2021 20:30






History, 20.05.2021 20:30