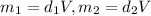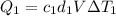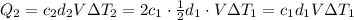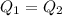You have two perfectly insulated cups. one contains water and the other contains an equal volume of another liquid that has half the density of water and twice the specific heat capacity. you heat the water from 10ºc to 20ºc and other liquid from 80ºc to 90ºc. compare the amount of heat energy needed to raise the temperature of the other liquid to the amount needed to raise the temperature of the water.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2


Chemistry, 23.06.2019 00:30
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1
You know the right answer?
You have two perfectly insulated cups. one contains water and the other contains an equal volume of...
Questions


History, 16.04.2020 00:51

Mathematics, 16.04.2020 00:51


Social Studies, 16.04.2020 00:51




Mathematics, 16.04.2020 00:51



Business, 16.04.2020 00:51

Chemistry, 16.04.2020 00:51


Mathematics, 16.04.2020 00:51

History, 16.04.2020 00:51


Mathematics, 16.04.2020 00:51

Social Studies, 16.04.2020 00:51

Mathematics, 16.04.2020 00:51

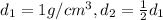 ;
;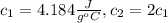 ;
;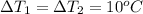 ;
;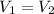 .
.