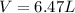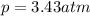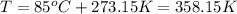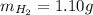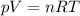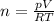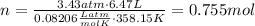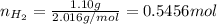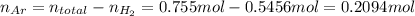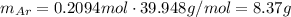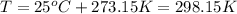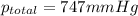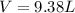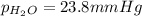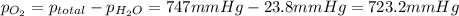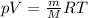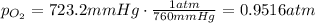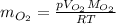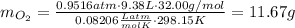Chemistry, 24.12.2019 18:31 yousifgorgees101
Amixture of hydrogen and argon gases is maintained in a 6.47 l flask at a pressure of 3.43 atm and a temperature of 85 °c. if the gas mixture contains 1.10 grams of hydrogen, the number of grams of argon in the mixture is g.
b) oxygen gas can be prepared by heating potassium chlorate according to the following equation:
2kclo3(s) > 2kcl(s) + 3o2(g)
the product gas, o2, is collected over water at a temperature of 25 °c and a pressure of 747 mm hg. if the wet o2 gas formed occupies a volume of 9.38l, the number of grams of o2 formed is g. the vapor pressure of water is 23.8 mm hg at 25 °c.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1

Chemistry, 22.06.2019 05:30
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 22.06.2019 10:30
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3
You know the right answer?
Amixture of hydrogen and argon gases is maintained in a 6.47 l flask at a pressure of 3.43 atm and a...
Questions

History, 08.01.2020 21:31
















Social Studies, 08.01.2020 21:31

Social Studies, 08.01.2020 21:31