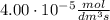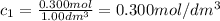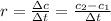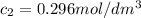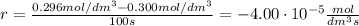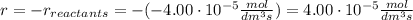Chemistry, 24.12.2019 18:31 hesterkl1225
At the start of a reaction, a 1.00 dm solution contains 0.300 mol of ethanol.
after 100 seconds the concentration of the ethanol has decreased to 0.296 mol/dmº
what is the rate of reaction over the first 100 seconds?
a 2.96 x 10-3 mol/dm/s
b 3.00 x 10 mol/dm/s
c 4.00 x 10 mol/dm®/s
d 8.00 x 10 mol/dm/s

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3

Chemistry, 23.06.2019 01:30
Some molecular compounds, such as hcl, ionize completely in solution. for molecular compounds such as h2co3, most molecules do not ionize in solution. which describes the properties of these two solutes? a. hcl and h2co3 have the same effect on the properties of the solution. b. hcl raises the freezing point of water more than h2co3 does. c. hcl raises the boiling point of water more than h2co3 does.
Answers: 2

Chemistry, 23.06.2019 07:30
Which statement is actually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 2
You know the right answer?
At the start of a reaction, a 1.00 dm solution contains 0.300 mol of ethanol.
after 100 seconds...
after 100 seconds...
Questions




History, 06.05.2020 20:58


Health, 06.05.2020 20:58




Mathematics, 06.05.2020 20:58


Physics, 06.05.2020 20:58

Mathematics, 06.05.2020 20:58

English, 06.05.2020 20:58


Mathematics, 06.05.2020 20:58

Mathematics, 06.05.2020 20:58


Physics, 06.05.2020 20:58