
In general, the value of the equilibrium constant for a chemical reaction does not depend
a) the temperature of the reaction vessel.
b) the initial amounts of reactants present.
c) the total pressure of the reaction vessel.
d) the volume of the reaction vessel.
e) the rate constants of the forward and reverse reactions.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:50
Which statement describes how phase changes can be diagrammed as a substance is heated? the phase is on the y-axis and the temperature is on the x-axis. the temperature is on the y-axis and the phase is on the x-axis. the time is on the y-axis and the temperature is on the x-axis. the temperature is on the y-axis and the time is on the x-axis.
Answers: 1

Chemistry, 23.06.2019 00:30
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1

Chemistry, 23.06.2019 07:20
Which of the following are acids or bases? 1. sodium hydrogen 2. barium hydroxide solution 3. carbonate solution
Answers: 1

Chemistry, 23.06.2019 10:00
Compare and contrast an assemblage and a pollen fingerprint by defying both and giving examples of each from the chapter.
Answers: 3
You know the right answer?
In general, the value of the equilibrium constant for a chemical reaction does not depend
Questions

Mathematics, 20.09.2020 08:01


Mathematics, 20.09.2020 08:01

Mathematics, 20.09.2020 08:01

English, 20.09.2020 08:01


English, 20.09.2020 08:01

Mathematics, 20.09.2020 08:01

Mathematics, 20.09.2020 08:01










English, 20.09.2020 08:01

English, 20.09.2020 08:01

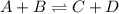
![K_{eq} = [C][D]](/tpl/images/0431/8264/aecc9.png)


