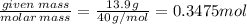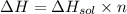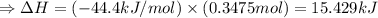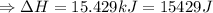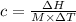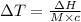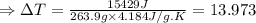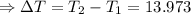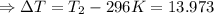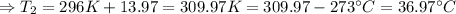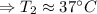Chemistry, 24.12.2019 02:31 coolman5999alt
The enthalpy of solution of sodium hydroxide is –44.4 kj/mol. when a 13.9-g sample of naoh dissolves in 250.0 g of water 23.0 °c in a coffee-cup calorimeter, what is the final temperature of the solution assuming no heat is lost to the surroundings. the solution has the same specific heat of 4.184 j/g-k.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2
You know the right answer?
The enthalpy of solution of sodium hydroxide is –44.4 kj/mol. when a 13.9-g sample of naoh dissolves...
Questions

Social Studies, 15.12.2020 14:50












Mathematics, 15.12.2020 14:50

Medicine, 15.12.2020 14:50


Arts, 15.12.2020 14:50

Medicine, 15.12.2020 14:50

Social Studies, 15.12.2020 14:50

Biology, 15.12.2020 14:50

Social Studies, 15.12.2020 14:50

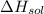 = – 44.4 kJ/mol,
= – 44.4 kJ/mol,