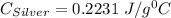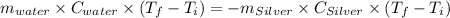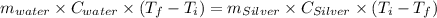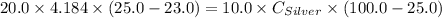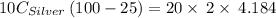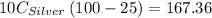Chemistry, 24.12.2019 02:31 markipler01
A10.0 gram sample of silver is heated to 100.0 degree c and then added to 20.0 g of water at 23.0 c, in an insulated container. at thermal equilibrium, the temperature of the system was measured and found to be 25.0 c. what is the specific heat, cs, of silver?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:10
For which one of the following reactions is the value of δh° rxn equal to δh° f for the product? a. 2 h2 (g) + o2 (g) → 2 h2o (l) b. n2 (g) + o2 (g) → 2 no (g) c. 2 h2 (g) + o2 (g) → 2 h2o (g) d. h2o (l) + 1/2 o2 (g) → h2o2 (l) e. none of the above
Answers: 1

Chemistry, 21.06.2019 22:10
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2

Chemistry, 22.06.2019 06:30
The minerals found in bones are deposited by living cells called
Answers: 1

Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1
You know the right answer?
A10.0 gram sample of silver is heated to 100.0 degree c and then added to 20.0 g of water at 23.0 c,...
Questions

Chemistry, 19.02.2021 23:10

English, 19.02.2021 23:10



Mathematics, 19.02.2021 23:10


Mathematics, 19.02.2021 23:10



History, 19.02.2021 23:10



Mathematics, 19.02.2021 23:10

English, 19.02.2021 23:10

Mathematics, 19.02.2021 23:10