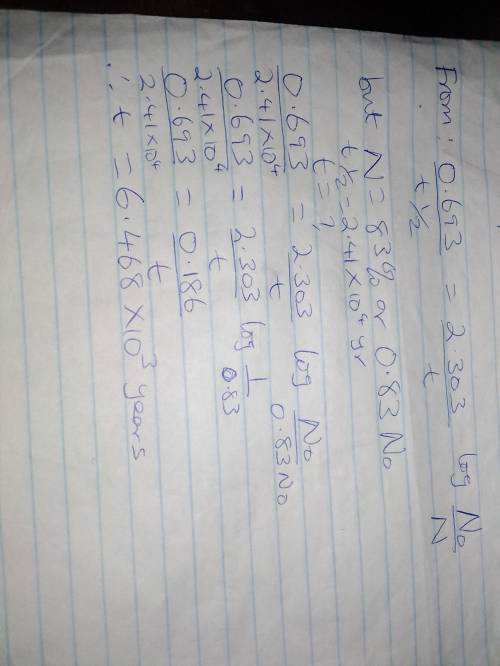Chemistry, 24.12.2019 01:31 biancaalegriashaffer
Even though plutonium−239 (t1/2 = 2.41 × 104 yr) is one of the main fission fuels, it is still a radiation hazard present in spent uranium fuel from nuclear power plants. how many years does it take for 83% of the plutonium−239 in spent fuel to decay?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 23.06.2019 01:50
Drag the tiles to the correct locations. each tile can be used more than once, but not all tiles will be used. one or more locations will remain empty. nitrosyl fluoride has the chemical formula nof nitrogen has five valence electrons, oxygen has six, and fluorine has seven. complete the lewis structure for this covalent compound. f n = = = . : : 0 : reset next um. all rights reserved us 2
Answers: 2

Chemistry, 23.06.2019 07:00
Select the correct answer. why are scientific models important in the study of science? a. they always involve critical mathematical calculations. b. they scientists understand complex ideas and objects that aren’t easy to handle. c. they enable scientists to popularize their work in society. d. they are required when conducting any peer review process. e. they are necessary for turning a hypothesis into a law.
Answers: 2

You know the right answer?
Even though plutonium−239 (t1/2 = 2.41 × 104 yr) is one of the main fission fuels, it is still a rad...
Questions

History, 13.10.2020 01:01

Computers and Technology, 13.10.2020 01:01


English, 13.10.2020 01:01

Geography, 13.10.2020 01:01

Chemistry, 13.10.2020 01:01

Mathematics, 13.10.2020 01:01

Mathematics, 13.10.2020 01:01



Mathematics, 13.10.2020 01:01

Biology, 13.10.2020 01:01

Mathematics, 13.10.2020 01:01

History, 13.10.2020 01:01


Mathematics, 13.10.2020 01:01

Biology, 13.10.2020 01:01

Social Studies, 13.10.2020 01:01