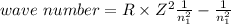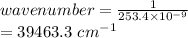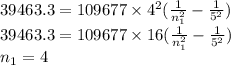Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Now consider the reaction when 45.0 g naoh have been added. what amount of naoh is this, and what amount of fecl3 can be consumed by it?
Answers: 3


Chemistry, 22.06.2019 21:30
If 22.5 of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. what is the new volume?
Answers: 1

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1
You know the right answer?
One of the emission spectral lines for be3+ has a wavelength of 253.4 nm for an electronic transitio...
Questions


Physics, 24.11.2020 20:00




Geography, 24.11.2020 20:00


Mathematics, 24.11.2020 20:00

English, 24.11.2020 20:00



Mathematics, 24.11.2020 20:00


Mathematics, 24.11.2020 20:00

Biology, 24.11.2020 20:00

Mathematics, 24.11.2020 20:00

Mathematics, 24.11.2020 20:00

Biology, 24.11.2020 20:00