
Chemistry, 23.12.2019 20:31 serenityarts123
Consider an ideal gas enclosed in a 1.00 l container at an internal pressure of 18.0 atm. calculate the work, w , if the gas expands against a constant external pressure of 1.00 atm to a final volume of 18.0 l.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1

Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2

Chemistry, 22.06.2019 23:30
Aweight lifter raises a 1600 n barbell to a height of 2.0 meters. how much work was done? w = fd a) 30 joules b) 3000 joules c) 320 joules d) 3200 joules
Answers: 2
You know the right answer?
Consider an ideal gas enclosed in a 1.00 l container at an internal pressure of 18.0 atm. calculate...
Questions

Mathematics, 17.05.2021 06:10


Mathematics, 17.05.2021 06:10

English, 17.05.2021 06:10


Mathematics, 17.05.2021 06:10



Mathematics, 17.05.2021 06:10

English, 17.05.2021 06:10

Mathematics, 17.05.2021 06:10

Mathematics, 17.05.2021 06:10






Mathematics, 17.05.2021 06:10

English, 17.05.2021 06:10

Advanced Placement (AP), 17.05.2021 06:10

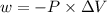
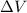 is the change in volume
is the change in volume
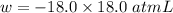
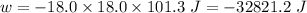 (negative sign implies that work is done by the system)
(negative sign implies that work is done by the system)

