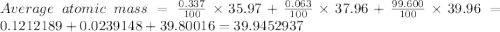Chemistry, 23.12.2019 19:31 kaydrama2003
Calculate the average atomic mass of argon to two decimal places, given the following relative atomic masses and abundances of each of the isotopes: argon-36 (35.97 u; 0.337%), argon-38 (37.96 u; 0.063%), and argon-40 (39.96 u; 99.600%)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:50
Which best describes why nh4+ can form an ionic bond with cl-?
Answers: 3


Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 09:30
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1
You know the right answer?
Calculate the average atomic mass of argon to two decimal places, given the following relative atomi...
Questions

English, 10.01.2022 04:00


Computers and Technology, 10.01.2022 04:00

Social Studies, 10.01.2022 04:00


Mathematics, 10.01.2022 04:00

Chemistry, 10.01.2022 04:00


History, 10.01.2022 04:00

Mathematics, 10.01.2022 04:00





Business, 10.01.2022 04:00


Chemistry, 10.01.2022 04:00


Engineering, 10.01.2022 04:00

English, 10.01.2022 04:00