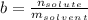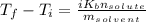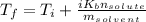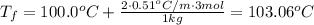Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

Chemistry, 22.06.2019 21:20
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3
You know the right answer?
What would the final boiling point of water be if 3 mol of nacl were added to 1 kg of water (k b =0....
Questions

History, 20.07.2019 13:10




Mathematics, 20.07.2019 13:10


Social Studies, 20.07.2019 13:10





Business, 20.07.2019 13:20


Biology, 20.07.2019 13:20

Mathematics, 20.07.2019 13:20


Biology, 20.07.2019 13:20

History, 20.07.2019 13:20


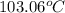
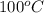 . When a salt is dissolved in a solvent, in this case water, it increases the boiling point of that solvent. The final boiling point can be calculated using the boiling point elevation formula which states that:
. When a salt is dissolved in a solvent, in this case water, it increases the boiling point of that solvent. The final boiling point can be calculated using the boiling point elevation formula which states that: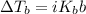
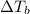 is the change in the boiling point, defined as:
is the change in the boiling point, defined as: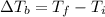
 is known as the van 't Hoff factor, in case we have a non-electrolyte/non-ionic substance, it's equal to 1, however, NaCl (aq) dissociates into 1 mole of sodium and 1 mole of chloride ions, so we have a total of 2 moles of ions per 1 mole of NaCl (aq), meaning i = 2, as the problem states;
is known as the van 't Hoff factor, in case we have a non-electrolyte/non-ionic substance, it's equal to 1, however, NaCl (aq) dissociates into 1 mole of sodium and 1 mole of chloride ions, so we have a total of 2 moles of ions per 1 mole of NaCl (aq), meaning i = 2, as the problem states;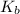 is known as the boiling point elevation constant for the solvent;
is known as the boiling point elevation constant for the solvent;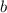 is the molality of substance, which is found dividing moles of solute by the kilograms of solvent:
is the molality of substance, which is found dividing moles of solute by the kilograms of solvent: