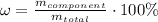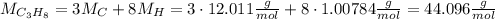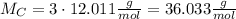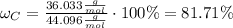Chemistry, 22.12.2019 22:31 haileyrae187
11. propane (c3hg) is a fuel commonly used in gas grills.
list the steps you would use to calculate the percent carbon in propane by mass.
the first step is started for you. (hint: my explanation required five steps.)
step 1: list what is known and unknown.
known: each mole of propane contains 3 moles c and 8 moles h.
unknown:
step 2:

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 23.06.2019 06:00
•what conclusions can you make about the relationship between the volume of a gas and its temperature? • what conclusions can you make about the relationship between the volume of a gas and its pressure? • what possible variables have you not accounted for? as you did the procedures, is it possible that the atmospheric pressure may have changed? if it did change over the course of your experiment, then how would your results have been affected?
Answers: 3

Chemistry, 23.06.2019 09:00
Which factor is likely to impact the possible number of compounds? presence of unlimited number of elements in the periodic table the inability of atoms to align perfectly with other atoms the ability of all elements to react with every other element all elements being equally reactive
Answers: 2
You know the right answer?
11. propane (c3hg) is a fuel commonly used in gas grills.
list the steps you would use to calc...
list the steps you would use to calc...
Questions

English, 24.11.2019 18:31

English, 24.11.2019 18:31

Mathematics, 24.11.2019 18:31






Physics, 24.11.2019 18:31

Mathematics, 24.11.2019 18:31





History, 24.11.2019 18:31

Mathematics, 24.11.2019 18:31


Biology, 24.11.2019 18:31


Computers and Technology, 24.11.2019 18:31

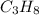 . In order to calculate the percent of carbon in propane by mass, we need to remember that %w/w (or percent mass) formula states that:
. In order to calculate the percent of carbon in propane by mass, we need to remember that %w/w (or percent mass) formula states that: