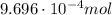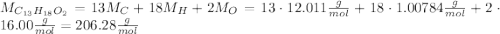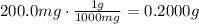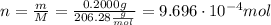Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 01:00
The primary products of complete combustion of fossil fuels are a. carbon dioxide and water b. methane and water c. carbon monoxide and water d. carbon dioxide and carbon monoxide
Answers: 1

Chemistry, 23.06.2019 02:00
Now look at the segment of the graph between the two data points marked with black squares. describe how the boiling point and melting point plots behave between these points. be as specific as possible.
Answers: 1

Chemistry, 23.06.2019 06:10
How much would the freezing point of water decrease if 4 mol of nacl were added to 1 kg of water (kf=1.86 degrees c/(mol/kg) for water and i=2 for nacl a- 7.44 degrees c b- 14.88 c 3.72 d 1.86
Answers: 1
You know the right answer?
ibuprofen (c13h1802) is the active ingredient in many nonprescription pain relievers. each tablet co...
Questions





Mathematics, 26.08.2019 00:00



History, 26.08.2019 00:00

Mathematics, 26.08.2019 00:00

Mathematics, 26.08.2019 00:00

Biology, 26.08.2019 00:00


Mathematics, 26.08.2019 00:00



History, 26.08.2019 00:00




Computers and Technology, 26.08.2019 00:00