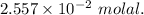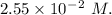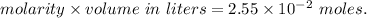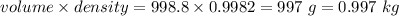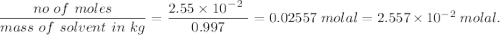Chemistry, 21.12.2019 06:31 genyjoannerubiera
A2.550 x 10^−2 m solution of glycerol (c3h8o3) in water is at 20.0°c. the sample was created by dissolving a sample of c3h8o3 in water and then bringing the volume up to 1.000 l. it was determined that the volume of water needed to do this was 998.9 ml . the density of water at 20.0°c is 0.9982 g/ml.
a. calculate the molality of the glycerol solution.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
The boiling point of liquids is very high what does it indicate
Answers: 1

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2

Chemistry, 22.06.2019 23:30
With the largest atoms and the smallest number of valence electrons and with the smallest atoms and the greatest number of valence electrons are the most reactive. a. nonmetals; metals b. nonmetals; transition elements c. transition elements; metals d. metals; nonmetals
Answers: 3

Chemistry, 23.06.2019 01:30
Ariver current has a velocity of 5km/h relative to the shore, and a boat moves in the same direction as the current at 5 km/h relative to the river. how can the velocity of the boat relative to the shore be calculated?
Answers: 1
You know the right answer?
A2.550 x 10^−2 m solution of glycerol (c3h8o3) in water is at 20.0°c. the sample was created by diss...
Questions

History, 22.01.2020 19:31

Chemistry, 22.01.2020 19:31





Mathematics, 22.01.2020 19:31


Social Studies, 22.01.2020 19:31

Mathematics, 22.01.2020 19:31

Mathematics, 22.01.2020 19:31

Health, 22.01.2020 19:31






English, 22.01.2020 19:31


Chemistry, 22.01.2020 19:31