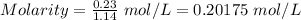Chemistry, 21.12.2019 05:31 snikergrace
At a particular temperature, k = 1.00×102 for the reaction: h2(g) + f2(g)= 2hf(g) in an experiment, at this temperature, 1.40 mol of h2 and 1.40 mol of f2 are introduced into a 1.14-l flask and allowed to react. at equilibrium, all species remain in the gas phase. what is the equilibrium concentration (in mol/l) of h2?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What is the empirical formula of vanadium 1 oxide given that 20.38 grams of vandium combines with oxygen to form 23.58 grams of the oxide
Answers: 1

Chemistry, 21.06.2019 23:00
City a and city b had two different temperatures on a particular day. on that day, four times the temperature of city a was 8â° c more than 3 times the temperature of city b. the temperature of city a minus twice the temperature of city b was â’3â° c. what was the temperature of city a and city b on that day? city a was 5â° c, and city b was 4â° c. city a was 3â° c, and city b was â’1â° c. city a was 8â° c, and city b was â’3â° c. city a was 5â° c, and city b was â’5â° c.
Answers: 2

Chemistry, 21.06.2019 23:00
Achef makes salad dressing by mixing oil, vinegar, and spices, as shown. which type of matter is the salad dressing?
Answers: 1

Chemistry, 22.06.2019 11:00
Problem page combustion of hydrocarbons such as pentane ( c5 h12 ) produces carbon dioxide, a "greenhouse gas." greenhouse gases in the earth's atmosphere can trap the sun's heat, raising the average temperature of the earth. for this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide.(a) write a balanced chemical equation, including physical state symbols, for the combustion of liquid pentane into gaseous carbon dioxide and gaseous water. (b) suppose 0.350 kg of pentane are burned in air at a pressure of exactly 1 atm and a temperature of 20.0 degree c. calculate the volume of carbon dioxide gas that is produced.be sure your answer has the correct number of significant digits.
Answers: 2
You know the right answer?
At a particular temperature, k = 1.00×102 for the reaction: h2(g) + f2(g)= 2hf(g) in an experiment,...
Questions


Mathematics, 19.11.2020 17:20


Mathematics, 19.11.2020 17:20

Mathematics, 19.11.2020 17:20

History, 19.11.2020 17:20

Physics, 19.11.2020 17:20



Mathematics, 19.11.2020 17:20



Mathematics, 19.11.2020 17:20

Mathematics, 19.11.2020 17:20


Mathematics, 19.11.2020 17:20



Mathematics, 19.11.2020 17:20

Spanish, 19.11.2020 17:20

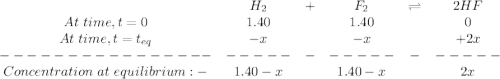
![K_c=\frac{[HF]^2}{[H_2][F_2]}](/tpl/images/0428/7279/a2854.png)
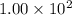
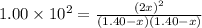
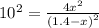
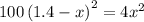
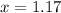
![[H_2]_{eq}=1.40-x=1.40-1.17=0.23\ moles](/tpl/images/0428/7279/ecf31.png)