Chemistry, 21.12.2019 04:31 MrRandomUser
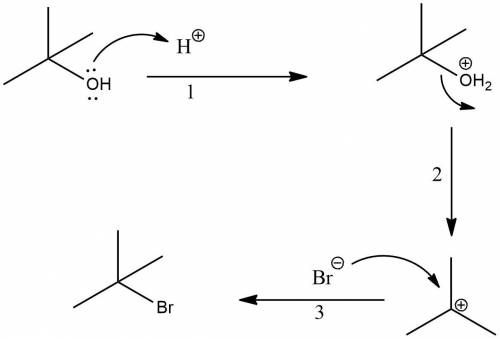
T-butyl bromide (2-bromo-2-methylpropane) can be prepared by simply taking t-butyl alcohol and shaking it with an aqueous solution of hbr at room temperature. the reaction is much faster than with n-butyl alcohol and is essentially 100% complete within a few minutes. give a mechanism for this reaction. what is this type of reaction called

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3

Chemistry, 22.06.2019 22:30
What is a number added in front of a formula in order to balance the equation
Answers: 1

Chemistry, 23.06.2019 01:00
Which statement characterizes synthetic polymers? a. they come from animals and plants. b. they are found in nature. c. they are made in a lab. d. they are components of starch.
Answers: 1
You know the right answer?
T-butyl bromide (2-bromo-2-methylpropane) can be prepared by simply taking t-butyl alcohol and shaki...
Questions

History, 09.01.2021 01:00

History, 09.01.2021 01:00

English, 09.01.2021 01:00


Chemistry, 09.01.2021 01:00


Mathematics, 09.01.2021 01:00


Mathematics, 09.01.2021 01:00




Mathematics, 09.01.2021 01:00

Chemistry, 09.01.2021 01:00

Mathematics, 09.01.2021 01:00

History, 09.01.2021 01:00


Mathematics, 09.01.2021 01:00

Mathematics, 09.01.2021 01:00

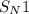 (substitution nucleophilic bimolecular) reaction.
(substitution nucleophilic bimolecular) reaction.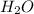 .
.