Chemistry, 21.12.2019 00:31 deziraynacole1960
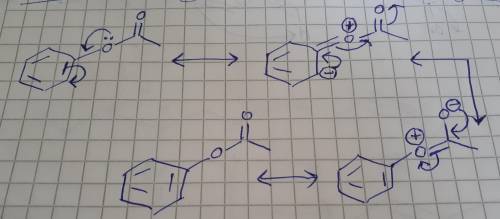
Draw a resonance structure, complete with all formal charges and lone (unshared) electron pairs, that shows the resonance interaction of the acetoxy with the ortho position in phenyl acetate. i. you do not have to consider stereochemistry. ii. include all valence lone pairs in your answer. iii. in cases where there is more than one answer, just draw one.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 20:40
Select the correct value for the indicated bond angle in each of the compounds. o−o−oo−o−o angle of o3 90° 109.5° < 109.5° 120° < 120° 180° f−b−ff−b−f angle of bf3 180° < 109.5° < 120° 120° 109.5° 90° f−o−ff−o−f angle of of2 < 120° 120° 90° 109.5° 180° < 109.5° cl−be−clcl−be−cl angle of becl2 90° 109.5° 180° 120° < 109.5° < 120° f−p−ff−p−f angle of pf3 90° 109.5° < 109.5° 180° 120° < 120° h−c−hh−c−h angle of ch4 90° < 109.5° 180° 120° < 120° 109.5°
Answers: 1


Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 2

Chemistry, 23.06.2019 04:10
Two solids are mixed in a flask and stirred. after a few minutes, the flask becomes cold. which of the following best describes this reaction? a. an exothermic reaction b. a combustion reaction c. an endothermic reaction d. a decomposition reaction
Answers: 1
You know the right answer?
Draw a resonance structure, complete with all formal charges and lone (unshared) electron pairs, tha...
Questions

Mathematics, 24.04.2020 20:04

Biology, 24.04.2020 20:04




Mathematics, 24.04.2020 20:04


Mathematics, 24.04.2020 20:04

Chemistry, 24.04.2020 20:04


World Languages, 24.04.2020 20:04

Social Studies, 24.04.2020 20:04



Social Studies, 24.04.2020 20:04




Mathematics, 24.04.2020 20:04