Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
If you want to create an electrical current, which situation would produce a solution capable of this
Answers: 3

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 23.06.2019 07:00
Determine the length of the object shown. 97.8 mm 97.80 mm 97 mm 98 mm
Answers: 1

Chemistry, 23.06.2019 10:30
The element chlorine has two stable isotopes, chlorine-35 with a mass of 34.97 amu and chlorine-37 with a mass of 36.95 amu. from the atomic weight of cl = 35.45 one can conclude that:
Answers: 2
You know the right answer?
Determine the value of kc for the following reaction if the equilibrium concentrations are as follow...
Questions




Biology, 13.09.2019 18:30











Biology, 13.09.2019 18:30


Biology, 13.09.2019 18:30



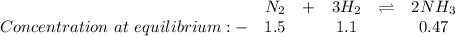
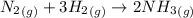
![K_c=\frac{[NH_3]^2}{[N_2][H_2]^3}](/tpl/images/0428/1906/c3aa0.png)