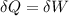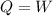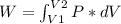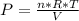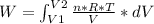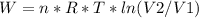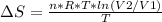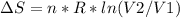Chemistry, 20.12.2019 19:31 Mattixwillard
An ideal gas contained in a piston-cylinder assembly is compressed isothermally in an internally reversible process.
(a) determine if the entropy change of the gas is greater than, equal to or less than zero, justify your answer
(b) determine if for the same change of state, the entropy change for an irreversible process is greater than, equal to or less than part (a)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
This is a mixture that has the same composition throughout.
Answers: 1

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3

Chemistry, 23.06.2019 01:00
Animals that reproduce sexually either do it through external or internal fertilization. read the following statement and decide if it is true or false. birds reproduce through external reproduction which is because the female will then be able to protect the egg.
Answers: 1
You know the right answer?
An ideal gas contained in a piston-cylinder assembly is compressed isothermally in an internally rev...
Questions

Chemistry, 08.11.2020 03:40

Mathematics, 08.11.2020 03:40

English, 08.11.2020 03:40




English, 08.11.2020 03:40


English, 08.11.2020 03:40




Biology, 08.11.2020 03:40

Mathematics, 08.11.2020 03:40


Computers and Technology, 08.11.2020 03:40




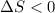
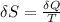
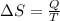
![\delta U=[tex]\delta Q- \delta W](/tpl/images/0427/9845/795a2.png)
![0=[tex]\delta Q- \delta W](/tpl/images/0427/9845/24655.png)