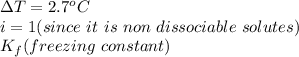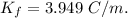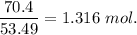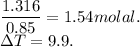Chemistry, 20.12.2019 19:31 savyblue1724707
When 70.4 g of benzamide (c7h7no) are dissolved in 850. g of a certain mystery liquid x , the freezing point of the solution is 2.7°c lower than the freezing point of pure x. on the other hand, when 70.4 g of ammonium chloride (nh4ci) are dissolved in the same mass of x, the freezing point of the solution is 9.9 °c lower than the freezing point of pure x.
a) calculate the van't hoff factor for ammonium chloride in x. be sure your answer has a unit symbol, if necessary, and round your answer to 2 significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

Chemistry, 22.06.2019 19:30
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1

Chemistry, 23.06.2019 00:00
How many atoms or molecules are there in a mole of a substance?
Answers: 1

Chemistry, 23.06.2019 01:00
Which substance—wood or silver—is the better thermal conductor? a thermal conductor is a material that requires very little heat energy to change its temperature. explain your answer.
Answers: 3
You know the right answer?
When 70.4 g of benzamide (c7h7no) are dissolved in 850. g of a certain mystery liquid x , the freezi...
Questions


Mathematics, 12.06.2020 15:57


Geography, 12.06.2020 15:57

English, 12.06.2020 15:57


Mathematics, 12.06.2020 15:57

Mathematics, 12.06.2020 15:57


Chemistry, 12.06.2020 15:57

Biology, 12.06.2020 15:57








History, 12.06.2020 15:57

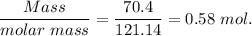
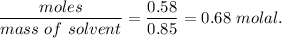
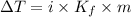 .....1
.....1