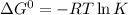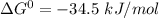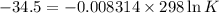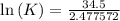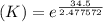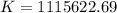Determine the equilibrium constant for the following reaction at 298 k.
cl(g) + o3(g) → clo(g)...

Chemistry, 20.12.2019 18:31 alexabbarker9781
Determine the equilibrium constant for the following reaction at 298 k.
cl(g) + o3(g) → clo(g) + o2(g)
δg° = - 34.5 kj

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Smog is the term used to describe the combination of fog and smoke
Answers: 1

Chemistry, 22.06.2019 00:00
The pressure in a fluid is affected by which characteristics of that fluid
Answers: 1


Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1
You know the right answer?
Questions


Physics, 20.01.2020 02:31

Chemistry, 20.01.2020 02:31


Biology, 20.01.2020 02:31

Mathematics, 20.01.2020 02:31


Mathematics, 20.01.2020 02:31

History, 20.01.2020 02:31

History, 20.01.2020 02:31


History, 20.01.2020 02:31



Physics, 20.01.2020 02:31



History, 20.01.2020 02:31


Health, 20.01.2020 02:31