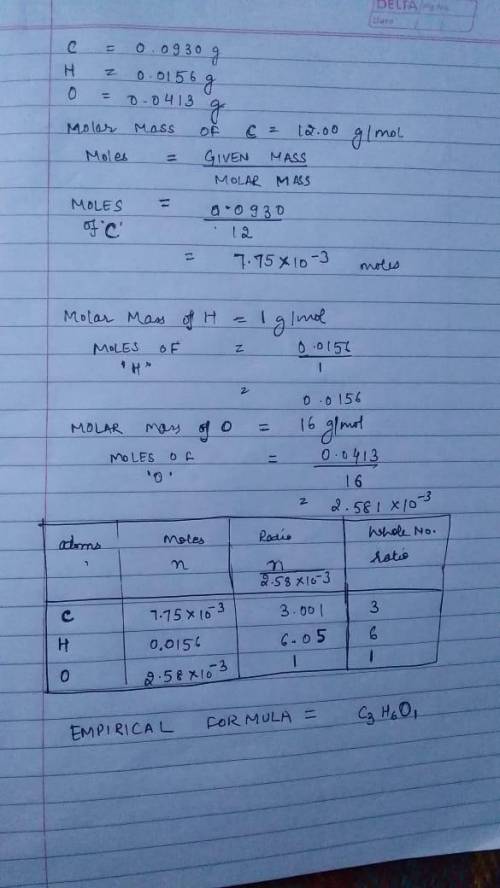
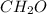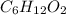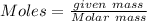Caproic acid, which is responsible for the foul odor of dirty socks, is composed of carbon, hydrogen, and oxygen atoms. a sample taken from a pair of abandon socks found in the locker room after a football game yielded 0.0930g of carbon, 0.0156g of hydrogen, and 0.0413g of oxygen. eww. they must have been worn more than once before being washed. find the empirical formula of caproic acid.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Agas has a volume of 0.7 l at 300 mmhg. what would be the new volume at 900 mmhg
Answers: 1

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2

Chemistry, 23.06.2019 00:00
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1

Chemistry, 23.06.2019 03:10
Which is true according to the law of conservation of energy
Answers: 1
You know the right answer?
Caproic acid, which is responsible for the foul odor of dirty socks, is composed of carbon, hydrogen...
Questions



Spanish, 23.06.2019 07:30

History, 23.06.2019 07:30











Computers and Technology, 23.06.2019 07:30


Biology, 23.06.2019 07:30

Chemistry, 23.06.2019 07:30


History, 23.06.2019 07:30

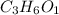
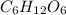 is
is