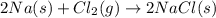Which of the following statements is not true?
a) when two nonmetals react, the compound formed is ionic.
b) two nonmetals can undergo an oxidation-reduction reaction.
c) a metal-nonmetal reaction can always be assumed to be an oxidation-reduction reaction.
d) a metal-nonmetal reaction involves electron transfer.
e) when a metal reacts with a nonmetal, an ionic compound is formed.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Amass of 100.0 g of solute is dissolved in water so that 850. ml of a 0.7500 m solution has been prepared. what is the molar mass of the solute?
Answers: 2

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1
You know the right answer?
Which of the following statements is not true?
a) when two nonmetals react, the compound for...
a) when two nonmetals react, the compound for...
Questions



Mathematics, 23.02.2021 18:00

Mathematics, 23.02.2021 18:00





History, 23.02.2021 18:00

Physics, 23.02.2021 18:00






Mathematics, 23.02.2021 18:00


History, 23.02.2021 18:00