Chemistry, 20.12.2019 03:31 brutalgitaffe
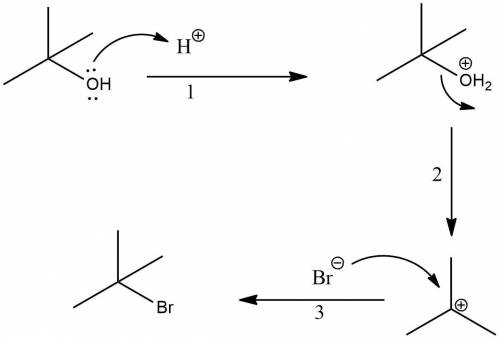
Butyl bromide (2-brom-2-methylpropane) can be prepared by simply taking t-butyl alcohol and shaking it with an aqueous solution of hbr at room temperature. the reaction is much faster than with n-butyl alcohol and is essentially 100% complete within a few minutes. give a mechanism for this reaction. note: it is not the same mechanism as for the lab preparation of 1-bromobutane. what is this reaction called?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:50
7. what temperature is need to just dissolve 50 g of nh4cl in 75 g of water? '
Answers: 1

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

You know the right answer?
Butyl bromide (2-brom-2-methylpropane) can be prepared by simply taking t-butyl alcohol and shaking...
Questions

Mathematics, 23.10.2019 16:50

History, 23.10.2019 16:50



Mathematics, 23.10.2019 16:50

Mathematics, 23.10.2019 16:50




History, 23.10.2019 16:50

English, 23.10.2019 16:50

Mathematics, 23.10.2019 16:50



Mathematics, 23.10.2019 16:50


English, 23.10.2019 16:50

English, 23.10.2019 16:50


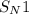 (substitution nucleophilic bimolecular) reaction
(substitution nucleophilic bimolecular) reaction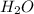 .
.