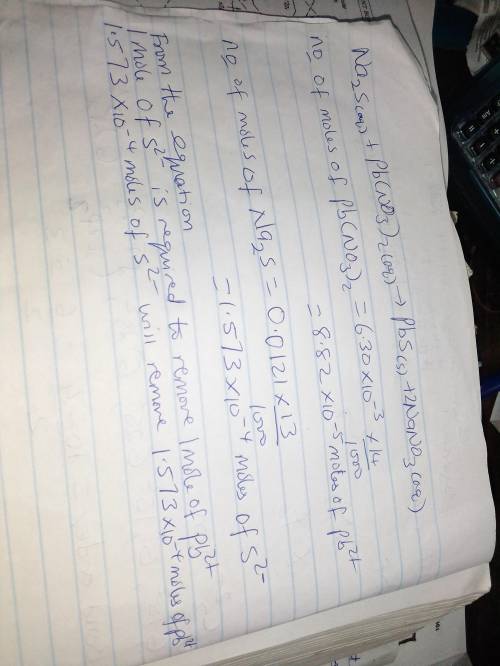Chemistry, 20.12.2019 02:31 sascsl2743
Heavy metal ions like lead(ii) can be precipitated from laboratory wastewater by adding sodium sulfide, na2s. will all the lead be removed from 14.0 ml of 6.30×10-3 m pb(no3)2 upon addition of 13.1 ml of 0.0121 m na2s? if all the lead is removed, how many moles of lead is this? if not, how many moles of pb remain?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
What are the charges of the subatomic particles by choosing the answer from the drop down menu. protons have a (+1,0,or-1). (protons,neutrons,electrons) have a 0 charge. 3.) electrons have a (+1,0,-1)
Answers: 2

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1
You know the right answer?
Heavy metal ions like lead(ii) can be precipitated from laboratory wastewater by adding sodium sulfi...
Questions

Mathematics, 06.03.2020 17:32

Social Studies, 06.03.2020 17:32



Mathematics, 06.03.2020 17:33





Mathematics, 06.03.2020 17:33







English, 06.03.2020 17:34


Social Studies, 06.03.2020 17:34

Mathematics, 06.03.2020 17:34