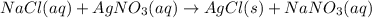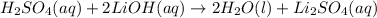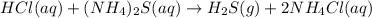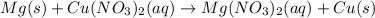Chemistry, 20.12.2019 02:31 wyattgrubb00
Nacl(aq) agno3(aq) → agcl(s) nano3(aq) : nacl(aq) agno3(aq) \rightarrow agcl(s) nano3(aq) : blank h2so4(aq) 2 lioh(aq) → 2 h2o(l) li2so4(aq) : h2so4(aq) 2 lioh(aq) \rightarrow 2 h2o(l) li2so4(aq) : blank hcl(aq) (nh4)2s(aq) → h2s(g) 2 nh4cl(aq) : hcl(aq) (nh4)2s(aq) \rightarrow h2s(g) 2 nh4cl(aq) : blank mg(s) cu(no3)2(aq) → mg(no3)2(aq) cu(s) :

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Two atoms interact with each other as shown by the equation. complete the equation by filling in the missing parts. 1 2 3 4 5 h he li
Answers: 2

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1

Chemistry, 22.06.2019 20:30
A40 kilogram skier starts at the top of a 12 meter high slope. at the bottom, she is travelling 10 meters per second. how much energy does she lose to friction
Answers: 2

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3
You know the right answer?
Nacl(aq) agno3(aq) → agcl(s) nano3(aq) : nacl(aq) agno3(aq) \rightarrow agcl(s) nano3(aq) : blank...
Questions

Biology, 01.10.2019 10:00


History, 01.10.2019 10:00


Arts, 01.10.2019 10:00


Chemistry, 01.10.2019 10:00


Physics, 01.10.2019 10:00

Chemistry, 01.10.2019 10:00

Mathematics, 01.10.2019 10:00






Chemistry, 01.10.2019 10:00

History, 01.10.2019 10:00

History, 01.10.2019 10:00