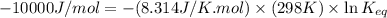Chemistry, 20.12.2019 01:31 maelaysiap
Carbon monoxide (co) is toxic because it binds more strongly to the iron in hemoglobin (hb) than does oxygen (o2), as indicated by these approximate standard free-energy changes in blood:
reaction a: reaction b: hb+o2hb+co⟶⟶hbo2,hbco, δg∘=−70 kj/mol δg∘=−80 kj/mol
estimate the equilibrium constant k at 298 k for the equilibrium
hbo2+co⇌hbco+o2

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Aroller coaster car is traveling down a track at 22 m/s. the car has a mass of 2000 kg. what is the kinetic energy of the car? a) 22,000 j b) 968,000 j c) 484,000 j d) 44,000 j
Answers: 2

Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1

Chemistry, 22.06.2019 03:30
Each pair of clay balls represents to planetesimals if each plane test molluscum pound of the same material and is separated by the same distance which pair experiences the greatest gravitational attraction
Answers: 2

Chemistry, 22.06.2019 07:30
Plz mark brainliest 30 points 1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s. 2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1
You know the right answer?
Carbon monoxide (co) is toxic because it binds more strongly to the iron in hemoglobin (hb) than doe...
Questions





Computers and Technology, 02.12.2021 21:30

Mathematics, 02.12.2021 21:30

Mathematics, 02.12.2021 21:30






History, 02.12.2021 21:30





Arts, 02.12.2021 21:30


Biology, 02.12.2021 21:30

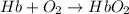 ;
; 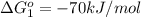
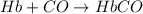 ;
; 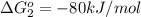
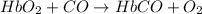 ;
; 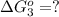
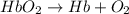 ;
; 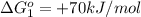
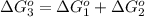
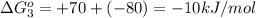
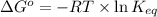
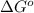 = standard Gibbs free energy = -10kJ/mol = -10000 J/mol
= standard Gibbs free energy = -10kJ/mol = -10000 J/mol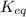 = equilibrium constant = ?
= equilibrium constant = ?