Chemistry, 20.12.2019 00:31 ashleypere99
Consider the reactions _1 2 n2(g) + _1 2 o2(g) → no(g) _1 2 n2(g) + o2(g) → no2(g) if these reactions come to equilibrium after combustion in an internal-combustion engine at 2000 k and 200 bar, estimate the mole fractions of no and no2 present for mole fractions of nitrogen and oxygen in the combustion products of 0.70 and 0.05.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3


Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 22.06.2019 17:30
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1
You know the right answer?
Consider the reactions _1 2 n2(g) + _1 2 o2(g) → no(g) _1 2 n2(g) + o2(g) → no2(g) if these reaction...
Questions


Advanced Placement (AP), 10.03.2021 18:20

Mathematics, 10.03.2021 18:20

Mathematics, 10.03.2021 18:20

Mathematics, 10.03.2021 18:20

Mathematics, 10.03.2021 18:20






Geography, 10.03.2021 18:20

Physics, 10.03.2021 18:20


Chemistry, 10.03.2021 18:20


Mathematics, 10.03.2021 18:20

Biology, 10.03.2021 18:20


History, 10.03.2021 18:20

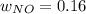

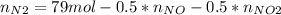
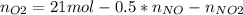
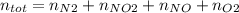
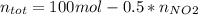
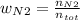
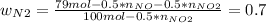
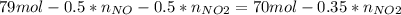
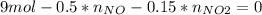 (1)
(1)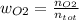
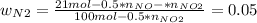
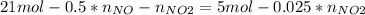
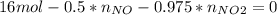 (2)
(2)