Chemistry, 19.12.2019 23:31 jdkrisdaimcc11
Calculate the value of e°cell for the following reaction: 2au(s) + 3ca2+(aq) → 2au3+(aq) + 3ca(s)au3+(aq) + 3e- → au(s) e° = 1.50vca2+(aq) + 2e- → ca(s) e° = -2.87va) -4.37 vb) -1.37 vc) -11.6 vd) 1.37 ve) 4.37 v

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 22.06.2019 22:30
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1

Chemistry, 23.06.2019 00:20
How many lone pairs of electrons are on the central atom of no3- and what is the molecular shape? one, trigonal planar zero, trigonal pyramidal zero, trigonal planar one, tetrahedral one, trigonal pyramidal
Answers: 1
You know the right answer?
Calculate the value of e°cell for the following reaction: 2au(s) + 3ca2+(aq) → 2au3+(aq) + 3ca(s)au3...
Questions





Chemistry, 13.12.2020 05:00



Mathematics, 13.12.2020 05:00



Mathematics, 13.12.2020 05:00


English, 13.12.2020 05:00


Mathematics, 13.12.2020 05:00



History, 13.12.2020 05:00

History, 13.12.2020 05:00

Mathematics, 13.12.2020 05:00

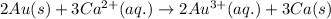
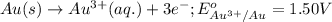 ( × 2 )
( × 2 )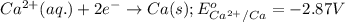 ( × 3 )
( × 3 )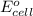 of the reaction, we use the equation:
of the reaction, we use the equation: