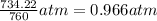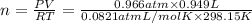The zinc within a copper-plated penny will dissolve in hydrochloric acid if the copper coating is filed down in several spots (so that the hydrochloric acid can get to the zinc). the reaction between the acid and the zinc is as follows: 2h+(aq)+zn(s)→h2(g)+zn2+(aq). when the zinc in a certain penny dissolves, the total volume of gas collected over water at 25 ∘c was 0.949 l at a total pressure of 758 mmhg .

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 22.06.2019 19:40
What is the wavelength of a 3*10^12 hz infrared wave a 3*10^20m b 1* 10^4m c 3*10^-3m d 1*10^-4 m
Answers: 1

Chemistry, 23.06.2019 00:50
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3

You know the right answer?
The zinc within a copper-plated penny will dissolve in hydrochloric acid if the copper coating is fi...
Questions


Mathematics, 13.05.2021 21:20


Social Studies, 13.05.2021 21:20

Mathematics, 13.05.2021 21:20


Mathematics, 13.05.2021 21:20


Physics, 13.05.2021 21:20

Social Studies, 13.05.2021 21:20

Spanish, 13.05.2021 21:20

Geography, 13.05.2021 21:20


Mathematics, 13.05.2021 21:20

Mathematics, 13.05.2021 21:20




Computers and Technology, 13.05.2021 21:20

Mathematics, 13.05.2021 21:20