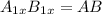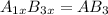Chemistry, 19.12.2019 20:31 clmorcutt420
In a hexagonal-close-pack (hcp) unit cell, the ratio of lattice points to octahedral holes to tetrahedral holes is 1: 1: 2.
what is the general formula for a compound if anions occupy the hcp lattice points and cations occupy all the octahedral holes? what is the general formula for a compound if anions occupy the hcp lattice points and cations occupy all the octahedral and tetrahedral holes?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3


Chemistry, 23.06.2019 00:30
What would be the original temperature of a gas that has a volume of 2.0 l and a pressure of 2.0 atm and an unknown temperature that the volume increased to 3.5 l in its pressure decreased to 1.0 atm if the final temperature is measured to be 11°c
Answers: 1

Chemistry, 23.06.2019 02:30
Ascientist wants to know how individual lions within a pride interact with each other in their own environment. to do this, the scientist sedates and tags all of the lions within a pride. then, he places several remotely-controlled video cameras near the lions' den and performs an observational field study. he collects continuous video footage over the span of one year, analyzes the video, and then forms conclusions based on his observations.
Answers: 2
You know the right answer?
In a hexagonal-close-pack (hcp) unit cell, the ratio of lattice points to octahedral holes to tetrah...
Questions

Biology, 20.10.2019 01:30

Mathematics, 20.10.2019 01:30

Mathematics, 20.10.2019 01:30




English, 20.10.2019 01:30

Physics, 20.10.2019 01:30




Mathematics, 20.10.2019 01:30


Mathematics, 20.10.2019 01:30



Mathematics, 20.10.2019 01:30

English, 20.10.2019 01:30

Biology, 20.10.2019 01:30