Chemistry, 19.12.2019 07:31 datzmypupppup
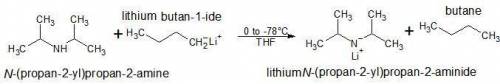
Lithium diisopropylamide is a strong, nonnucleophilic base. it is often freshly prepared by treating a certain reactant with n-butyllithium (n-buli). draw the starting material and draw the product (lithium diisopropylamide). include any charges, but you do not need to draw electron pairs.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

You know the right answer?
Lithium diisopropylamide is a strong, nonnucleophilic base. it is often freshly prepared by treating...
Questions



Mathematics, 02.07.2020 01:01



Mathematics, 02.07.2020 01:01

English, 02.07.2020 01:01

Mathematics, 02.07.2020 01:01

Mathematics, 02.07.2020 01:01

Mathematics, 02.07.2020 01:01


Mathematics, 02.07.2020 01:01



Mathematics, 02.07.2020 01:01

English, 02.07.2020 01:01

Mathematics, 02.07.2020 01:01


Mathematics, 02.07.2020 01:01