Chemistry, 19.12.2019 06:31 priscillarios30
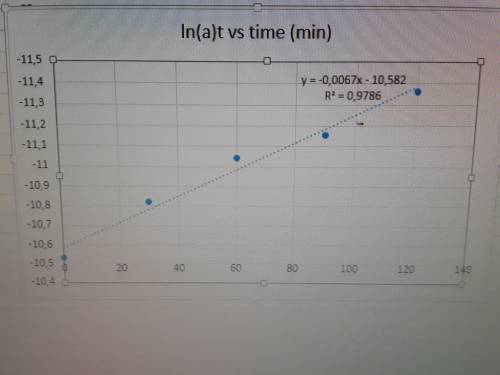
Acolored dye compound decomposes to give a colorless product. the original dye absorbs at 608 nm and has an extinction coefficient of 4.7 ×10⁴ m⁻¹cm⁻¹ at that wavelength.
you perform the decomposition reaction in a 1-cm cuvette in a spectrometer and obtain the following data:
time (min) - absorbance at 608 nm
0 - 1.254
30 - 0.941
60 - 0.752
90 - 0.672
120 - 0.545
from these data, determine the rate law for the reaction "dye → product" and determine the rate constant.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Balance and in which category does it fit in? single or double displacement or synthesis or decomposition? (a) k2 o → k + o2 (b) na + i2 → nai (c) cu(no3 )2 + naoh → cu(oh)2 + nano3 (d) kclo3 → kcl + o2 (e) ca(no3 )2 + hbr → cabr2 + hno3 (f) sn(oh)2 → sno + h2 o (g) p4 + n2 o → p4 o6 + n2 (h) fe + al2 (so4 )3 → feso4 + al (i) alcl3 + na2 co3 → al2 (co3 )3 + nacl (j) c3 h6 + o2 → co2 + h2 o
Answers: 1

Chemistry, 22.06.2019 23:30
Aweight lifter raises a 1600 n barbell to a height of 2.0 meters. how much work was done? w = fd a) 30 joules b) 3000 joules c) 320 joules d) 3200 joules
Answers: 2

Chemistry, 23.06.2019 00:30
How can you write e method for the experiment of separating sand from water by filtration process? 1-materials 2-steps 3-conclusion also the same for the separating process of water and salt by filtration or distillation. quick because i need to finish my hw
Answers: 2

Chemistry, 23.06.2019 02:50
Dumbledore decides to gives a surprise demonstration. he starts with a hydrate of na2co3 which has a mass of 4.31 g before heating. after he heats it he finds the mass of the anhydrous compound is found to be 3.22 g. he asks everyone in class to determine the integer x in the hydrate: na2co3·xh2o; you should do this also. round your answer to the nearest integ
Answers: 2
You know the right answer?
Acolored dye compound decomposes to give a colorless product. the original dye absorbs at 608 nm and...
Questions

Biology, 28.09.2020 20:01


Computers and Technology, 28.09.2020 20:01


Mathematics, 28.09.2020 20:01

Chemistry, 28.09.2020 20:01

Social Studies, 28.09.2020 20:01

History, 28.09.2020 20:01

Mathematics, 28.09.2020 20:01


English, 28.09.2020 20:01

Mathematics, 28.09.2020 20:01

Mathematics, 28.09.2020 20:01

Social Studies, 28.09.2020 20:01

History, 28.09.2020 20:01


Mathematics, 28.09.2020 20:01

English, 28.09.2020 20:01


Mathematics, 28.09.2020 20:01