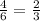Chemistry, 19.12.2019 01:31 asiababbie33
The al2o3 crystal structure (corundum) consists of an hcp arrangement of o2- ions; the al3+ ions occupy octahedral positions. what fraction of the available octahedral positions are filled with al3+ ions? in corundum, the al3+ ions do not fill the tetrahedral positions.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Aspirin has a density of 1.40 g/cm3 what is the volume in cubic centimeters of a tablet weighing 320 mg ?
Answers: 1



You know the right answer?
The al2o3 crystal structure (corundum) consists of an hcp arrangement of o2- ions; the al3+ ions oc...
Questions


History, 22.07.2019 02:01


Social Studies, 22.07.2019 02:01

Biology, 22.07.2019 02:01

Physics, 22.07.2019 02:01

Biology, 22.07.2019 02:01





Mathematics, 22.07.2019 02:01


Mathematics, 22.07.2019 02:01

English, 22.07.2019 02:01

English, 22.07.2019 02:01

Spanish, 22.07.2019 02:01


Mathematics, 22.07.2019 02:01

English, 22.07.2019 02:01

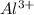 ions is
ions is 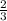
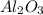 crystal structure consists of an HCP arrangement of
crystal structure consists of an HCP arrangement of 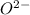 ions.
ions.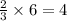
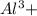 ions are present = 4
ions are present = 4