Chemistry, 19.12.2019 00:31 dgayles8761
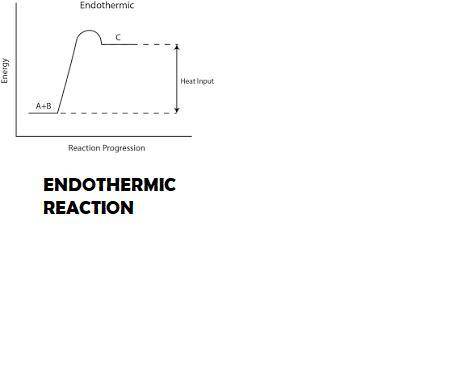
The dissolution of ammonium nitrate, nh4no3, in water is an endothermic process. since the calorimeter is not a perfect insulator, will the enthalpy of solution for ammonium nitrate be reported as too high or too low if this heat change is ignored? explain

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:20
Pegmatites are igneous rocks in which the individual minerals are very large. typically, the minerals are all light-colored quartz, feldspar and muscovite. if you were given a black and white photograph of a pegmatite in a quarry (where the rock has been blasted and broken), what physical properties could you use to identify those three minerals in this hypothetical photo? describe each mineral and the specific diagnostic properties. be specific.
Answers: 2

Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3

Chemistry, 23.06.2019 03:20
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3
You know the right answer?
The dissolution of ammonium nitrate, nh4no3, in water is an endothermic process. since the calorimet...
Questions

Biology, 28.10.2019 23:31

Mathematics, 28.10.2019 23:31

Chemistry, 28.10.2019 23:31

Chemistry, 28.10.2019 23:31



Mathematics, 28.10.2019 23:31


Mathematics, 28.10.2019 23:31

Geography, 28.10.2019 23:31



Mathematics, 28.10.2019 23:31


Mathematics, 28.10.2019 23:31



Chemistry, 28.10.2019 23:31


Biology, 28.10.2019 23:31