
Chemistry, 19.12.2019 00:31 bonnysvalentine
1. from the following standard electrochemical potential (and your knowledge of biomedically important metals), answer the questions below. reaction δeº(v) ti -> ti+3 2.00 al -> al+3 1.70 cr -> cr+2 0.56 h2-> 2h+ 0.00 au -> au+ -1.68 a. which metal would be the most anodic? b. which metal would corrode the most? c. which metal would be the most reactive? d. why can ti and au be used for making implants, but not al?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2


Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2
You know the right answer?
1. from the following standard electrochemical potential (and your knowledge of biomedically importa...
Questions



Mathematics, 02.03.2021 23:50

Mathematics, 02.03.2021 23:50


Mathematics, 02.03.2021 23:50

Mathematics, 02.03.2021 23:50


Mathematics, 02.03.2021 23:50


English, 02.03.2021 23:50



Mathematics, 02.03.2021 23:50





Mathematics, 02.03.2021 23:50

Mathematics, 02.03.2021 23:50

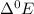 : Standard Reduction Potential : It is tendency of the element to gain electron and get reduced.
: Standard Reduction Potential : It is tendency of the element to gain electron and get reduced.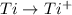 has maximum value of
has maximum value of 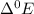 = 2.00V , so it get oxidised as compared to other elements.
= 2.00V , so it get oxidised as compared to other elements. 

