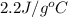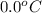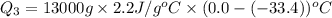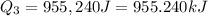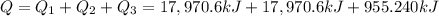Liquid ammonia, , was used as a refrigerant fluid before the discovery of the chlorofluorocarbons and is still widely used today. its normal boiling point is –33.4 °c, and its vaporization enthalpy is 23.5 kj/mol. the gas and liquid have specific heat capacities of 2.2 and 4.7 , respectively. calculate the heat energy transfer required to raise the temperature of 13.0 kg liquid ammonia from –50.0 °c to 0.0 °c. heat energy = kj

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Asample of radium-226 will decay 1/4 of its original amount after 3200years. what is the half-life of radium-226?
Answers: 2

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1
You know the right answer?
Liquid ammonia, , was used as a refrigerant fluid before the discovery of the chlorofluorocarbons an...
Questions

Mathematics, 13.07.2019 22:00

Mathematics, 13.07.2019 22:00



English, 13.07.2019 22:00

Mathematics, 13.07.2019 22:00





Biology, 13.07.2019 22:00



Chemistry, 13.07.2019 22:00

Mathematics, 13.07.2019 22:00

Social Studies, 13.07.2019 22:00

Social Studies, 13.07.2019 22:00

Arts, 13.07.2019 22:00


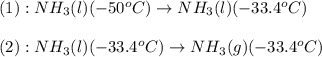
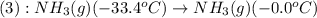

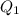 = amount of heat absorbed = ?
= amount of heat absorbed = ?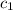 = specific heat of liquid ammonia =
= specific heat of liquid ammonia = 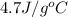
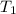 = initial temperature =
= initial temperature = 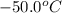
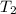 = final temperature =
= final temperature = 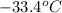
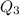 , we get:
, we get: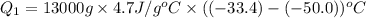
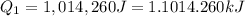
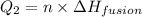
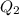 = amount of heat absorbed = ?
= amount of heat absorbed = ?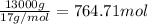
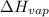 = enthalpy change for vaporization=23.5 kJ/mol
= enthalpy change for vaporization=23.5 kJ/mol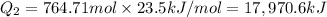
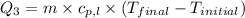
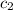 = specific heat of gaseous ammonia =
= specific heat of gaseous ammonia =