
Rusting of iron is a very common chemical reaction. it results in one form from fe reacting with oxygen gas to produce iron (iii) oxide. your sample of iron is 12.0 moles of iron. so which if these is a true statement? note: all numbers located immediately after elemental symbols below should be considered subscripts. a. 4.5 moles of o2 and produce 3.0 moles of fe2o3. b. 12.0 moles of o2 and produce 24.0 moles of fe2o3. c. 9.0 moles of o2 and produce 3.0 moles of fe2o3. d. 9.0 moles of o2 and produce 6.0 moles of fe2o3 e. none of the above

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1
You know the right answer?
Rusting of iron is a very common chemical reaction. it results in one form from fe reacting with oxy...
Questions

History, 16.07.2019 20:30

History, 16.07.2019 20:30

History, 16.07.2019 20:30

History, 16.07.2019 20:30

History, 16.07.2019 20:30


Mathematics, 16.07.2019 20:30



History, 16.07.2019 20:30


Mathematics, 16.07.2019 20:30

History, 16.07.2019 20:30







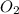 and produce 6.0 moles of
and produce 6.0 moles of 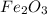
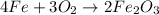
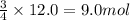 of oxygen gas
of oxygen gas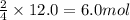 of iron (III) oxide
of iron (III) oxide

