

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 01:00
If a straight-chain hydrocarbon is a gas at room temperature, how many carbon atoms will it have? a. 6 carbon atoms b. 12 carbon atoms c. 24 carbon atoms d. 3 carbon atoms
Answers: 1

Chemistry, 23.06.2019 03:50
Which best describes the activation energy of a chemical reaction? a. the combined energy of all the reactants b. the amount of energy required for a reaction to occur c. the difference in energy between products and reactants d. the potential energy stored in the bonds of reactants and products
Answers: 1

Chemistry, 23.06.2019 07:40
What is the reduction potential of a hydrogen electrode that is still at standard pressure, but has ph = 5.65 , relative to the she?
Answers: 1

Chemistry, 23.06.2019 08:30
Kelly has come up with an explanation for why her sister is sometimes in a good mood and other times in a bad mood. she speculates that it is based on the hours of sleep her sister got the previous night. this explanation for her sister's behaviors is an example of a(n)
Answers: 3
You know the right answer?
If solid nacl is added to a saturated water solution of pbcl2 at 20o c, a precipitate is formed. how...
Questions

Mathematics, 18.12.2020 18:50

Mathematics, 18.12.2020 18:50

Mathematics, 18.12.2020 18:50

History, 18.12.2020 18:50

Business, 18.12.2020 18:50



Mathematics, 18.12.2020 18:50


English, 18.12.2020 18:50


English, 18.12.2020 18:50

Mathematics, 18.12.2020 18:50







History, 18.12.2020 18:50

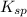 remains the same
remains the same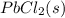 ⇄
⇄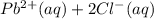
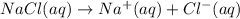
![K_{sp}=[Pb^{2+}][Cl^-]^2](/tpl/images/0424/5297/7fd11.png)
![S=[Pb^{2+}]=\frac{K_{sp}}{[Cl^-]^2}](/tpl/images/0424/5297/c2c31.png)


