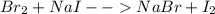Unknown halogen x2 was added to each of two test tubes which contained aqueous nacl and aqueous nai, respectively. when hexane was added to the test tubes, an orange color developed in the top layer of the first test tube, while purple developed in the second.
a. identify x2.
b. write a balanced equation for each reaction which occurred.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1


Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

You know the right answer?
Unknown halogen x2 was added to each of two test tubes which contained aqueous nacl and aqueous nai,...
Questions


Biology, 23.04.2020 21:26


Mathematics, 23.04.2020 21:26




English, 23.04.2020 21:26

Chemistry, 23.04.2020 21:26







Mathematics, 23.04.2020 21:26




Mathematics, 23.04.2020 21:26

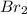
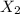 is
is 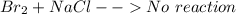 (because bromine is less reactive than chlorine)
(because bromine is less reactive than chlorine)