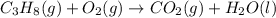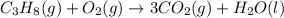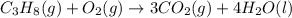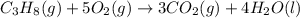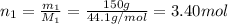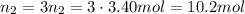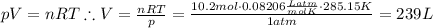Chemistry, 18.12.2019 18:31 kirstennnash
Combustion of hydrocarbons such as propane ( c3h8 ) produces carbon dioxide, a "greenhouse gas." greenhouse gases in the earth's atmosphere can trap the sun's heat, raising the average temperature of the earth. for this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide.
1. write a balanced chemical equation, including physical state symbols, for the combustion of gaseous propane into gaseous carbon dioxide and gaseous water.
2. suppose 0.150kg of propane are burned in air at a pressure of exactly 1atm and a temperature of 12.0°c . calculate the volume of carbon dioxide gas that is produced. round your answer to 3 significant digits. l

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2

Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2

Chemistry, 23.06.2019 01:00
Atoms contain subatomic particles called protons and neutrons. when these protons and neutrons spilt, a lot of energy is released
Answers: 3
You know the right answer?
Combustion of hydrocarbons such as propane ( c3h8 ) produces carbon dioxide, a "greenhouse gas." gre...
Questions

Mathematics, 30.01.2020 21:40

Mathematics, 30.01.2020 21:40


Biology, 30.01.2020 21:40






Biology, 30.01.2020 21:40

History, 30.01.2020 21:40






Biology, 30.01.2020 21:40

Mathematics, 30.01.2020 21:40