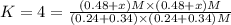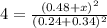Chemistry, 18.12.2019 05:31 djmelodiedaniels
When the reaction co2(g) + h2(g) ⇄ h2o(g) + co(g) is at equilibrium at 1800◦c, the equilibrium concentrations are found to be [co2] = 0.24 m, [h2] = 0.24 m, [h2o] = 0.48 m, and [co] = 0.48 m. then an additional 0.34 moles per liter of co2 and h2 are added. when the reaction comes to equilibrium again at the same temperature, what will be the molar concentration of co?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:10
There are 6.022 x 10^23 atoms of hg in 1 mole of hg. the number of atoms in 4.5 moles of hg can be found by multiplying 4.5 by 6.022 x 10^23 a. 2.7 x 10^24 b. 27 x 10^23 c. 2.71 x10^24 d. 27.099 x 10^23
Answers: 3

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 18:00
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3
You know the right answer?
When the reaction co2(g) + h2(g) ⇄ h2o(g) + co(g) is at equilibrium at 1800◦c, the equilibrium conce...
Questions

Mathematics, 22.01.2020 07:31



Mathematics, 22.01.2020 07:31


Mathematics, 22.01.2020 07:31



History, 22.01.2020 07:31

Mathematics, 22.01.2020 07:31


Chemistry, 22.01.2020 07:31


Geography, 22.01.2020 07:31



Mathematics, 22.01.2020 07:31



Mathematics, 22.01.2020 07:31

![[CO_2] = 0.24 M, [H_2] = 0.24 M, [H_2O] = 0.48 M, [CO] = 0.48 M](/tpl/images/0423/7365/45c13.png)
![K=\frac{[H_2O][CO]}{[CO_2][H_2]}=\frac{0.48 M\times 0.48 M}{0.24 M\times 0.24 M}](/tpl/images/0423/7365/2a444.png)
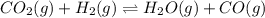
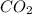 and
and 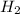 are added.
are added.