
Chemistry, 18.12.2019 04:31 broomssymphonie
There are three ways in which to define acids and bases: the arrhenius concept, the brønsted-lowry concept, and the lewis concept. arrhenius acids are substances that, when dissolved in water, increase the concentration of the h+ ion; arrhenius bases are substances that, when dissolved in water, increase the concentration of the oh− ion. brønsted-lowry acids are substances that can donate a proton (h+) to another substance; brønsted-lowry bases are substances that can accept a proton (h+). a lewis acid is an electron-pair acceptor, and a lewis base is an electron-pair donor. part a using the arrhenius concept of acids and bases, identify the arrhenius acid and base in each of the following reactions: 2koh(aq)+h2so4(aq)→k2so4(aq)+2h2o(l ) nh3(g)+hcl(g)→nh4cl(s)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Si una estrella no tiene paralaje medible, ¿qué puedes inferir?
Answers: 1

Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3

Chemistry, 23.06.2019 10:30
Fill in the blanks for the following statements: the rms speed of the molecules in a sample of h2 gas at 300 k will be times larger than the rms speed of o2 molecules at the same temperature, and the ratio µrms (h2) / µrms (o2) with increasing temperature. a not enough information is given to answer this question b sixteen, will not change c four, will not change d four, will increase e sixteen, will decrease
Answers: 2

Chemistry, 23.06.2019 14:30
Will give imagine you are given a mystery element. it is, however, a discovered and known element. you may perform a maximum of two observations or tests to determine its identity. time and money is critical, so you need to prioritize your tests. if you can get by with a single test, you get 100 super-geek points from your research lab team. pick your two tests, number them as #1 and #2, and justify why you think these two will certainly be enough (and why the first might well be enough all by itself.) the available tests are classification into metal, non-metal, or metalloid, count of valence electrons, count of electron shells, atomic radius (error range: +/- 1 pm), electronegativity (error range: +/- 0.1), first ionization energy (error range: +/- 10 kj/mole), melting point (error range: +/- 10 c), and boiling point (error range: +/- 20 c).
Answers: 2
You know the right answer?
There are three ways in which to define acids and bases: the arrhenius concept, the brønsted-lowry...
Questions







Business, 19.09.2019 00:00




Mathematics, 19.09.2019 00:00





Mathematics, 19.09.2019 00:00




Mathematics, 19.09.2019 00:00

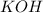 acts as base.
acts as base.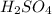 acts as acid.
acts as acid.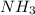 acts as base.
acts as base.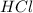 acts as acid.
acts as acid.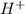 .
.
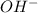 .
.

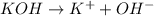 and hence, acts as base.
and hence, acts as base.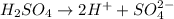 and hence, acts as acid.
and hence, acts as acid.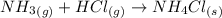
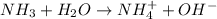 and hence, acts as base.
and hence, acts as base.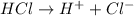 and hence, acts as acid.
and hence, acts as acid.

