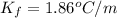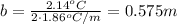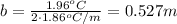Chemistry, 18.12.2019 04:31 carolyvillanueva
Asample of sea water taken from the atlantic ocean freezes at -2.14 degree celsius and a sample taken from the arctic ocean freezes at -1.96 degree celsius. what is the molality of salt in each seawater sample? (assume the only solute in each sample is sodium chloride)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 22.06.2019 23:00
What is the number of neutrons in an atom with atomic mass of 35
Answers: 2

Chemistry, 23.06.2019 07:00
Under what conditions will a gas be most likely to exhibit the ideal gas properties predicted by the ideal gas law? 1)high pressures and high temperature, because particles are forced closer together with higher kinetic energy, so intermolecular forces of attraction are weaker 2)high pressure and low temperature, because particles are forced closer together and moving slower, so the volume of the particles is less significant 3) low pressure and high temperature, because particles are spread farther apart and moving faster, so the intermolecular forces of attraction are weaker 4)low pressure and low temperature, because particles are spread farther apart with lower kinetic energy, so the volume of the particles is less significant
Answers: 2

Chemistry, 23.06.2019 07:30
Which statement is actually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 2
You know the right answer?
Asample of sea water taken from the atlantic ocean freezes at -2.14 degree celsius and a sample take...
Questions



Mathematics, 19.08.2019 14:30



Chemistry, 19.08.2019 14:30

Chemistry, 19.08.2019 14:30



Biology, 19.08.2019 14:30

Health, 19.08.2019 14:30

Biology, 19.08.2019 14:30


Physics, 19.08.2019 14:30


Mathematics, 19.08.2019 14:30



Biology, 19.08.2019 14:30

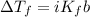
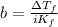
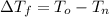
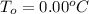 is the initial freezing point of water,
is the initial freezing point of water,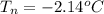 is the final freezing point of water.
is the final freezing point of water.