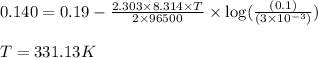An electrochemical cell is constructed such that on one side a pure nickel electrode is in contact with a solution containing ni2+ ions at a concentration of 3 × 10−3 m. the other cell half consists of a pure fe electrode that is immersed in a solution of fe2+ ions having a concentration of 0.1 m. at what temperature will the potential between the two electrodes be +0.140 v?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3

You know the right answer?
An electrochemical cell is constructed such that on one side a pure nickel electrode is in contact w...
Questions


Biology, 05.05.2020 17:02

English, 05.05.2020 17:02







History, 05.05.2020 17:02

Mathematics, 05.05.2020 17:02


Chemistry, 05.05.2020 17:02







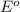 potential will always get reduced and will undergo reduction reaction.
potential will always get reduced and will undergo reduction reaction. 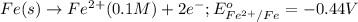
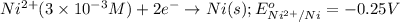
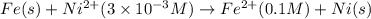
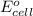 of the reaction, we use the equation:
of the reaction, we use the equation: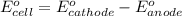
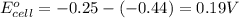
![E_{cell}=E^o_{cell}-\frac{2.303RT}{nF}\log \frac{[Fe^{2+}]}{[Ni^{2+}]}](/tpl/images/0423/5757/27fa9.png)
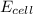 = electrode potential of the cell = +0.140 V
= electrode potential of the cell = +0.140 V![[Fe^{2+}]=0.1M](/tpl/images/0423/5757/e5f8e.png)
![[Ni^{2+}]=3\times 10^{-3}M](/tpl/images/0423/5757/de767.png)