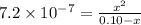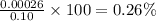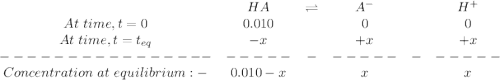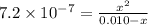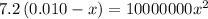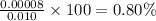Chemistry, 18.12.2019 01:31 mariamalakozay603
Percent ionization
percent ionization for a weak acid (ha) is determined by the following formula:
percent ionization=[ha] ionized[ha] initial×100%
for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.
a certain weak acid, ha, has a ka value of 7.6×10−7.
part a
calculate the percent ionization of ha in a 0.10 m solution.
express your answer as a percent using two significant figures.
%
submithintsmy answersgive upreview part
part b
calculate the percent ionization of ha in a 0.010 m solution.
express your answer as a percent using two significant figures.
%

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:10
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3


Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2

Chemistry, 23.06.2019 00:00
If many scientists conduct the same or similar experiments, and all obtain similar results, a can be written, which is a generally agreed-upon statement that explains and predicts how a natural phenomenon works.
Answers: 1
You know the right answer?
Percent ionization
percent ionization for a weak acid (ha) is determined by the following form...
percent ionization for a weak acid (ha) is determined by the following form...
Questions


Chemistry, 31.08.2019 13:30


Mathematics, 31.08.2019 13:30

Social Studies, 31.08.2019 13:30

History, 31.08.2019 13:30



English, 31.08.2019 13:30

Mathematics, 31.08.2019 13:30



Biology, 31.08.2019 13:30


Mathematics, 31.08.2019 13:30

Business, 31.08.2019 13:30

Biology, 31.08.2019 13:30

Chemistry, 31.08.2019 13:30


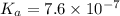
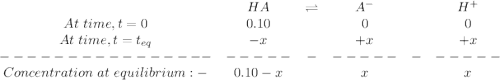
![K_{a}=\frac {\left [ H^{+} \right ]\left [ {A}^- \right ]}{[HA]}](/tpl/images/0423/3691/59869.png)