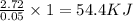Chemistry, 18.12.2019 01:31 mallorynichole19
When a student mixes 50 ml of 1.0 m hcl and 50 ml of 1.0 m naoh in a coffee-cup calorimeter, the temperature of the resultant solution increases from 21.0 °c to 27.5 °c. calculate the enthalpy change for the reaction in kj per mol of hcl, assuming that the calorimeter loses only a negligible quantity of heat. the total volume of the solution is 100 ml, its density is 1.0 g/ml, and its specific heat is 4.18 j/g*k.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 23.06.2019 03:30
If 2 molecules of one reactant combine with 3 molecules of another to produce 5 molecules of a product, then what is the representation of the reaction?
Answers: 1

Chemistry, 23.06.2019 12:30
Is the genie in the bottle experiment a physical or chemical change/reaction?
Answers: 1
You know the right answer?
When a student mixes 50 ml of 1.0 m hcl and 50 ml of 1.0 m naoh in a coffee-cup calorimeter, the tem...
Questions

Chemistry, 01.12.2021 22:30


Business, 01.12.2021 22:30


Chemistry, 01.12.2021 22:30

Mathematics, 01.12.2021 22:30


Mathematics, 01.12.2021 22:30





Mathematics, 01.12.2021 22:30

Computers and Technology, 01.12.2021 22:30

Mathematics, 01.12.2021 22:30


English, 01.12.2021 22:30

English, 01.12.2021 22:30

Mathematics, 01.12.2021 22:30

Biology, 01.12.2021 22:30

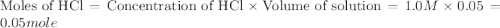
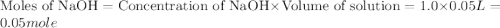

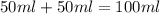
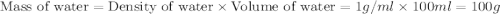
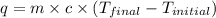
 = specific heat of water =
= specific heat of water = 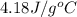
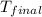 = final temperature of water =
= final temperature of water = 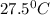
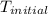 = initial temperature of metal =
= initial temperature of metal = 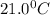
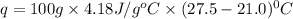
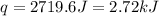
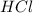 :
: