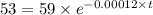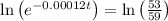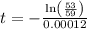The half life for the decay of carbon-14 is 5.73 x 10^3 years. suppose the activity due to the radioactive decay of the carbon-14 in a tiny sample of an artifact made of wood from an archeological dig is measured to be 53.bq. the activity in a similar-sized sample of fresh wood is measured to be 59.bq.
1. calculate the age of the artifact. round your answer to 2 significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:50
The name of the ion, s2-, is: sulfurous ion sulfide ion sulfur ion sulfate ion
Answers: 1

Chemistry, 22.06.2019 21:30
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2

Chemistry, 23.06.2019 04:10
An unknown substance has been shown to have metallic bonds. which of the following is most likely a property of this substance? a. low conductivity b. low boiling point c. high malleability d. high solubility in water
Answers: 2

Chemistry, 23.06.2019 05:00
1. true or false: minerals are inorganic. true false 2. inorganic means that something has never been found alive 3. halite is another name for and is a mineral with a cubic crystal pattern. table salt rock salt
Answers: 2
You know the right answer?
The half life for the decay of carbon-14 is 5.73 x 10^3 years. suppose the activity due to the radio...
Questions

Computers and Technology, 16.11.2020 17:00


Spanish, 16.11.2020 17:00


Business, 16.11.2020 17:00










English, 16.11.2020 17:00





Mathematics, 16.11.2020 17:00

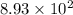 years
years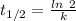
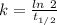
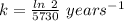
![[A_t]](/tpl/images/0423/2686/5262c.png) = 53 Bq
= 53 Bq![[A_t]=[A_0]e^{-kt}](/tpl/images/0423/2686/1ef89.png)