
Consider the following reaction:
2hcl(g) + 185 kj → h2(g) + cl2(g)
describe the change in energy according to the law of conservation of energy.
a) there is a net gain of 185 kj of energy when decomposing hcl.
b) it takes more energy to break the bonds of hcl than form h2(g) + cl2(g).
c) the energy required to decompose hcl and produce h2(g) + cl2(g) are equal.
d) it takes less energy to break the bonds of hcl than form the bonds of h2(g) + cl2(g.)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2


Chemistry, 23.06.2019 05:00
Scientists discovered fossils in several layers of the earth you see here. they found fossils of algae, snails, and clams in layer d. given that information, where do you think they found fossil evidence of simple land plants and amphibians?
Answers: 1
You know the right answer?
Consider the following reaction:
2hcl(g) + 185 kj → h2(g) + cl2(g)
describe the c...
2hcl(g) + 185 kj → h2(g) + cl2(g)
describe the c...
Questions



Mathematics, 26.02.2021 21:00



Mathematics, 26.02.2021 21:00



Mathematics, 26.02.2021 21:00



Mathematics, 26.02.2021 21:00


Mathematics, 26.02.2021 21:00


Chemistry, 26.02.2021 21:00


Mathematics, 26.02.2021 21:00


Social Studies, 26.02.2021 21:00

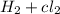 are equal.
are equal.

